How Far Out Is Your Pipeline?
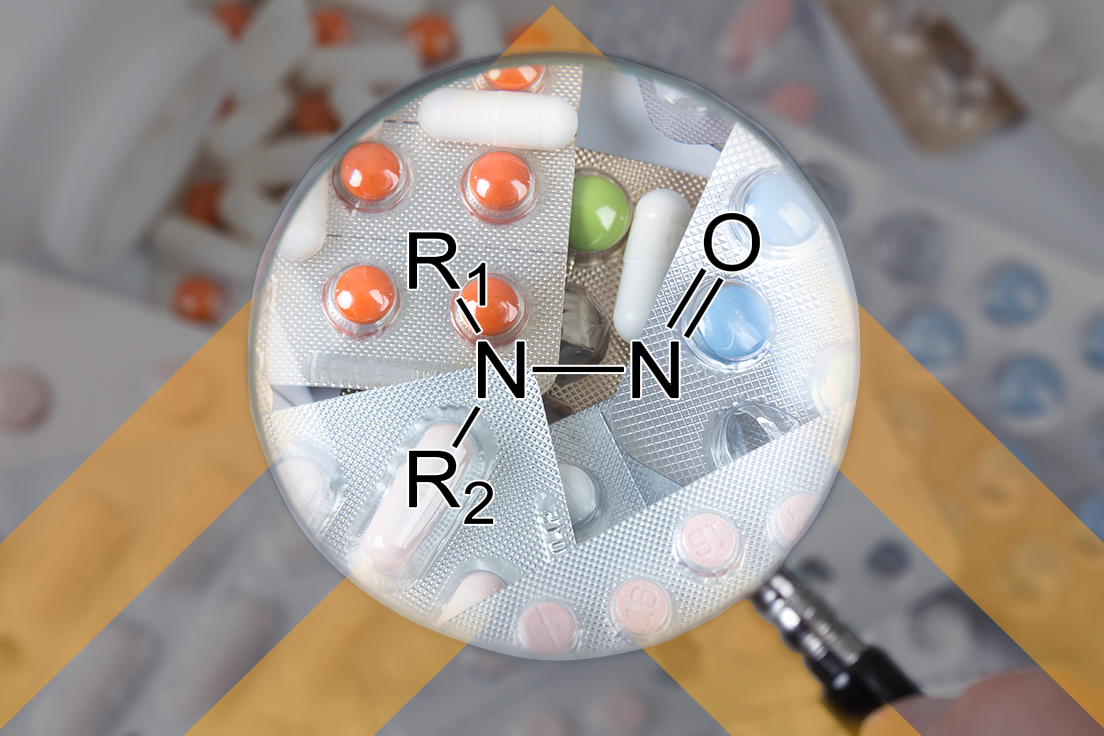
In the generic drug business, most times it is best to be first! This means trying to be first in development, submission, and (of course) approval. But, in order to have a chance of being first, you must know what to do, and that is especially true of knowing what the bioequivalence (BE) requirements are. […]