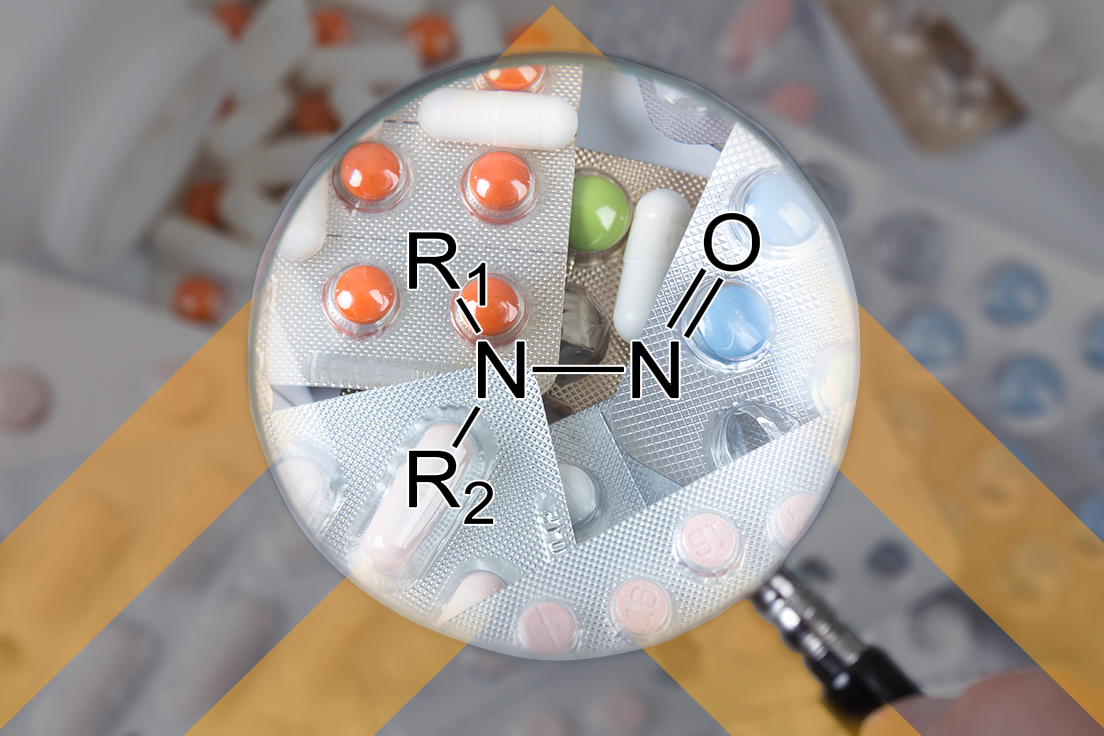
Nitrosamines – Where Are We Now?
Two years after the FDA guidance on Control of Nitrosamines Impurities in Human Drugs and nearly a year after the publication of USP’s General Chapter <1469> Nitrosamine Impurities, where are we now and what have we learned? That was a question posed recently at the Association of Accessible Medicine’s GRx+Biosims 2022 conference. The session devoted […]





